Quizzes > High School Quizzes > Science
Practice Quiz: Methods of Heat Transfer
Test Your Knowledge with Key Heat Concepts
Study Outcomes
- Identify and describe the three methods of heat transfer: conduction, convection, and radiation.
- Analyze how each method of heat transfer occurs in various physical contexts.
- Differentiate between the roles of conduction, convection, and radiation in thermal processes.
- Apply concepts of heat transfer to solve practice problems and real-life scenarios.
- Evaluate test preparation strategies based on targeted feedback from quiz performance.
Heat Transfer Quiz: 50 Methods Cheat Sheet
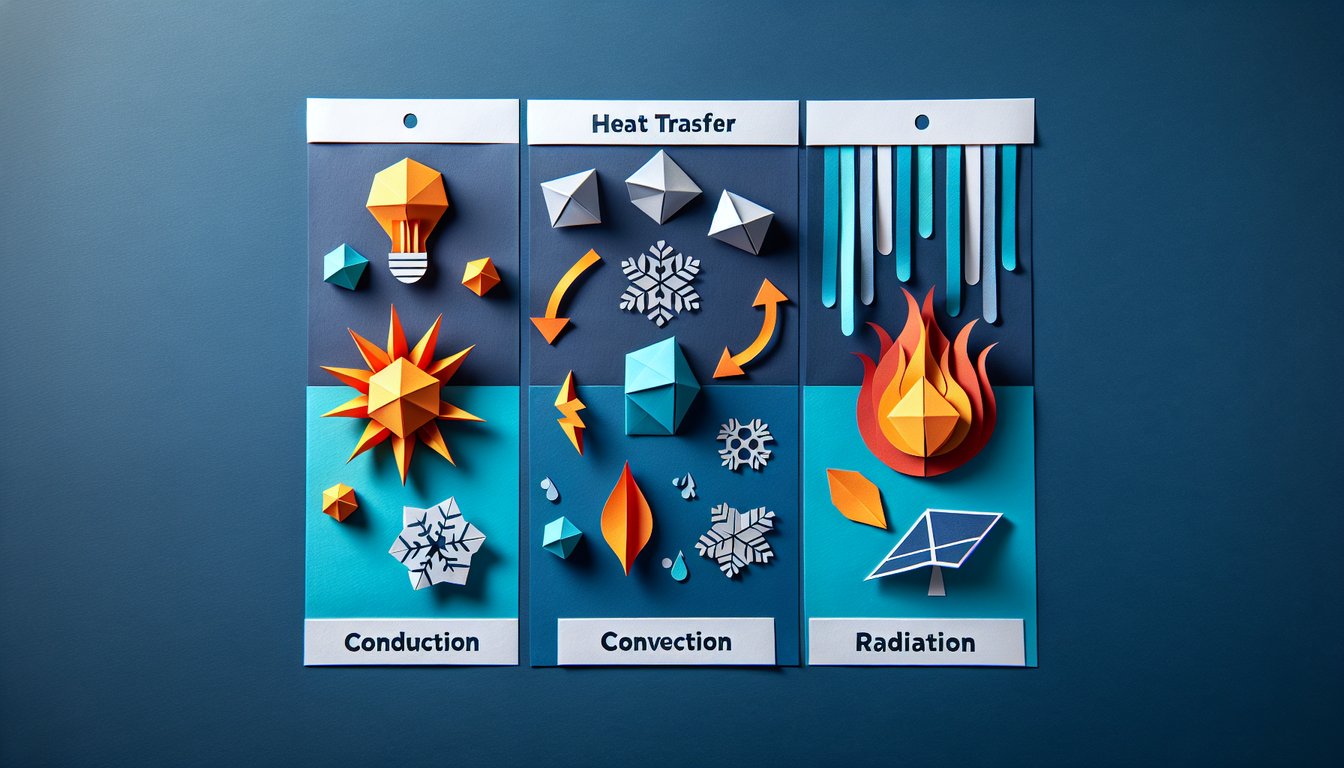
- Three Heat Transfer Methods - Heat energy moves around in three awesome ways: conduction, convection, and radiation. Mastering these methods is your ticket to understanding everything from how your coffee cools to why the Earth warms. Keep this overview handy as your go‑to roadmap! Learn more on OpenStax
- Conduction - Conduction happens when heat travels through direct contact, like a metal spoon warming up in hot tea. It's all about microscopic particles bumping into each other to pass along energy. Imagine an army of tiny billiard balls transferring momentum - pretty cool, right? Read the conduction deep dive
- Convection - Convection throws fluids into the mix, moving heat by circulating warmer, lighter fluid upward and letting cooler fluid sink. Think of boiling water or atmospheric winds - nature's own lava lamp! Understanding this helps you ace topics from weather to radiator design. Explore convection details
- Radiation - Radiation transfers heat without touching anything, using invisible electromagnetic waves. The cozy warmth you feel from the Sun or a campfire is pure radiative magic. No medium required - just waves cruising through space! Discover radiation principles
- Heat Transfer Equation (Q = mcΔT) - This formula is your calculation superhero: Q (heat energy) equals mass times specific heat capacity times temperature change. Plug in the numbers to find out how much energy you need to heat or cool a substance. Practicing with real‑life examples makes it stick! Practice with the equation
- Specific Heat Capacity - Specific heat capacity tells you how much heat is needed to raise 1 kg of material by 1 °C. Water wins gold here, requiring a ton of energy to warm up - perfect for temperature regulation on Earth (and in your tea!). Knowing this explains why different materials heat up at different rates. Unpack specific heat
- Thermal Conductivity - Thermal conductivity measures how fast materials conduct heat - metals zip heat away like speedy sprinters, while insulators like wood creep along slowly. That's why a metal doorknob feels icy in winter! This property is key for engineering everything from cookware to spacecraft. See conductivity in action
- Natural vs. Forced Convection - Natural convection relies on buoyancy as hot fluid rises and cool fluid sinks, whereas forced convection uses fans or pumps to get fluids moving. Picture gentle ocean currents versus a hairdryer blasting warm air. Both types are vital in HVAC, electronics cooling, and meteorology. Compare convection types
- Stefan‑Boltzmann Law - This law spells out how objects radiate heat: Q = σA(T❴ - T₂❴), where σ is the Stefan‑Boltzmann constant and A is surface area. It's the secret behind calculating heat loss from stars, planets, and engineering designs. Mastering it lets you predict radiative behavior like a physics pro! Learn the law details
- Everyday Applications - From designing efficient ovens and car engines to understanding weather patterns and climate control, heat transfer is everywhere in our daily lives. Spot these principles in action and you'll never look at a cup of coffee or a summer breeze the same way again! See real‑world examples